Monitoring Oesophagus Position from Intra-cardiac Echocardiography During Atrial Fibrillation Ablation
Left atrium (LA) posterior wall (LAPW) comprises essential targets for transcatheter radiofrequency ablation (RFA) of atrial fibrillation (AF), but poses problems due to a complex anatomy and to retro-atrial structures potentially damaged by RF, mostly the oesophagus and the related plexi and nerves. Apart from the dreaded atrio-oesophageal fistula, which is fortunately rare (<0.1%), oesophageal lesions have been described in 1.6-28% of cases, and endoscopic mucosal lesions are present in up to 20% of cases. No preventative method has gained wide acceptance yet. Intracardiac echocardiography (ICE) can be integrated with the 3D electro-anatomical maps constructed by CARTO system (Biosense Webster, Diamond Bar, CA, USA), and can give unique real-time anatomical information about all closely-located peri-cardiac structures. In clinical practice, however, the positions of LAPW and oesophagus are monitored by manually tracing ICE images which are transferred onto a 3D electro-anatomical map. This procedure is imprecise, cumbersome and time-consuming, and represents a freezed representation of a dynamic situation.
Accordingly, in this project we aim to automatically detect dynamic oesophagus position and its spatial relationship from the LAPW by ICE during RFA of AF.
 Figure 1
Figure 1
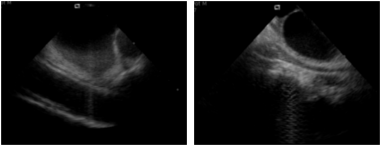 Figure 2
Figure 2
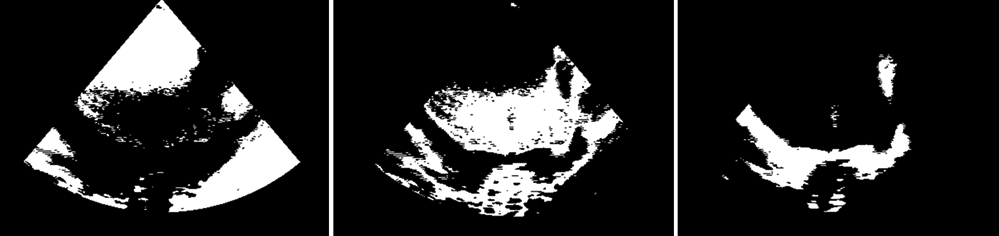 Figure 3
Figure 3
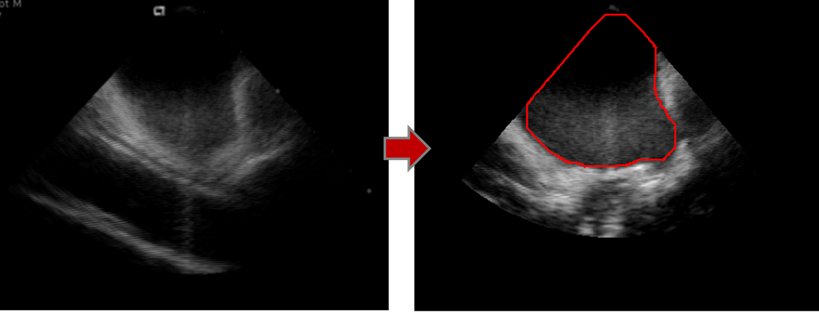 Figure 4
Figure 4
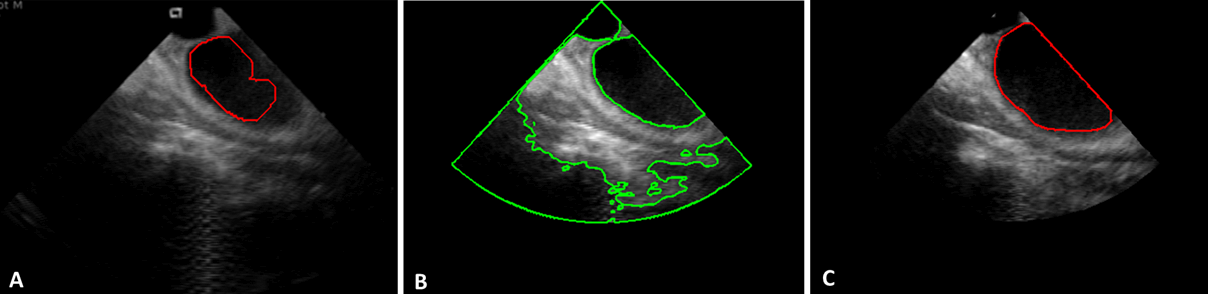 Figure 5
Figure 5
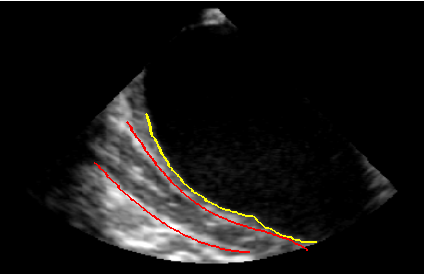 Figure 6
Figure 6
As a first step to track real-time posterior LA anatomy and retro-atrial spatial 3D relationships, LAPW was automatically detected. A mask corresponding to the echo scan was created to limit working area. Otsu’s method was applied to identify the darkest region within the mask (Figure 1A and 1B). The region depicting the LA was then automatically selected by exploiting LA shape and position knowledge. In fact, in this type of acquisition, LA is typically positioned in the up-right quarter of the echo scan. The centroid of the selected region was then computed (Figure 1C).
Following this step, depending on the amount of noise in the ICE sequence, two different segmentation methods were developed to detect the LAPW in each class of images.
To detect LA boundaries in very noisy images (Figure 2, left panel) a procedure based on clustering K-means algorithm (CL) was developed. Our hypothesis was to differentiate pixels corresponding to blood, cardiac structures and noisy regions by applying a clustering K-means method with k=3. Pixels belonging to the cluster with higher mean gray-level value were considered “cardiac structures”; pixels belonging to the cluster with the lower mean gray-level value were considered “blood” and the remaining pixels belonging to noisy regions (Figure 3).
The image was then segmented applying a threshold defined as a weighted average of cluster centers. The resulting detection was refined using morphological operators. The region containing the atrium centroid was selected and dilated with a structural element of the same size of the one used for the erosion. Finally, the detected LA cavity was refined by applying a boundary regularization procedure (Figure 4).
To detect LA boundaries in images affected by little noise (Figure 2, right panel), a procedure based on the region-based level-set Chan-Vese method (CV) was developed. The method was applied considering the boundary of the region obtained at the previous step as initial condition (Figure 5A). Result of CV-based segmentation is shown in figure 5B. The LA region was then selected by using the centroid position previously detected and refined by removing “holes”, applying erosion and dilatation steps with structural elements of the same size, together with a regularization step (Figure 5C).
Once LAPW was automatically detected, a fast algorithm based on the evaluation of gray-level intensity distribution in the image excluding the LA was developed to detect candidate pixels belonging to the oesophagus wall. Fitting of candidate pixels resulted in distal and proximal oesophagus boundaries (Figure 6).
Work in progress regards validation of the proposed approach and dynamic tracking and monitoring of LAPW and during AF RFA to prevent oesophagus injuries.








