A(FIB)2ROTIC
Marie Curie Fellow: Martino Alessandrini;
Host Institution: Department of Electrical, Electronic and Information Engineering, University of Bologna (Prof. S. Severi and Prof. C. Corsi);
Institution of secondment: Department of Computer Science, University of Oxford (Prof. B. Rodriguez)
AFFIBROTIC is a EU funded Marie Curie Individual fellowship (GA 659082) running between 1/10/2015 and 30/9/2017. The goal of AFIBROTIC was to study key patho-mechanisms of atrial fibrillation by using patient specific computational modelling.
AFIBROTIC on Cordis
Youtube channel
Clinical Context
Atrial fibrillation (AF) is the most common form of arrhythmia worldwide. In the EU, the prevalence of AF (2%) has doubled since the last decade and it is estimated that number of AF patients by 2030 will be between 14 and 17 million. AF is associated with substantial morbidity and mortality. In particular, patients with AF have a five-fold higher risk of stroke. Management of AF and AF-related complications has a high burden on the social security systems (~10bEUR in 2004).Despite the high clinical and societal priority, clinical management of AF is still suboptimal. AF is typically treated by pulmonary veins (PVs) isolation by catheter ablation. However, success rates of PV isolation in non-paroxysmal patients (i.e., roughly the two thirds of AF patients) are as low as 28% for a single procedure. Indeed, non-paroxysmal AF is sustained by complex electrical sources, such as rotors focal sources and multiple wavelets, which persist after PV isolation. Hereto, ablation schemes that address such electrical features (e.g. rotor-driven ablation) have gained popularity. Yet, the initial enthusiasm is mitigated by growing skepticism due to the difficulty in replicating such protocols in multicenter studies. Overall, ablation of non-paroxysmal AF remains an unstandardized procedure with low success rates (42%).
Moreover, stroke risk is treated either pharmaceutically (by anticoagulant drugs) or procedurally (by left clamping the left atrial appendage). Yet, stroke risk stratification indices, such as the CHADS score, are based on extremely generic parameters (e.g., age, hypertension, diabetes mellitus) and their predictive power remains low.
The overall objective of AFIBROTIC was therefore to study the key mechanisms of atrial fibrillation, in particular connected to electrical conduction and blood flow dynamics, by using patient-specific computational modelling. Computational modelling provides a unique framework to study the response of a biological system to given solicitations (boundary conditions) in a fully controlled reproducible and non-invasive way. Ultimately, the developed technology aims to leads toward personalized optimal therapy delivery for the atrial fibrillation patient.
A benchmarking framework for rotor-driven ablation of atrial fibrillation with multielectrode catheters
Pulmonary vein (PV) ablation is typically employed to treat atrial fibrillation (AF). Yet, further substrate ablation is often necessary. Hereto, Focal Impulse and Rotor Modulation (FIRM) guided ablation has raised great interest given the high reported success rates. Yet, the initial excitement was mitigated by a growing skepticism due to the difficulty in verifying the protocol in multicenter studies. The underlying assumptions of the FIRM protocol still need verification: i) AF is sustained by stable rotors; ii) a rotor can be terminated by ablating its core; iii) basket catheters (such as Constellation, Boston Scientific) allow reliable rotor mapping.Here, we use computational modelling to test some of the underlying assumptions and benchmark basket-guided ablation. In particular, we tested i) if the rotor core can be reliably localized by a grid of electrodes and how the number/resolution of electrodes and the distance from the atrial wall can affect such localization ii) if simulated ablation is able per se to terminate AF and if the spatial information derived from the EGMs is critical for the procedure success. This study is the result of a collaboration between the Department of Electrical, Electronic and Information Engineering at UNIBO (Bologna, IT), Institute of Biomedical Engineering at Karlsruhe Institute of Technology (Karlsruhe, DE) and Ospedale Bufalini (Cesena, IT).
The acCELLerate simulator (Karlsruhe Institute of Technology) was used to initiate reentry in four detailed atrial models.
Simulated reentry on a 3D atrial model. Output produced with acCELLerate, Kerlsruhe Institute of Technology.
From the activation sequence, the electrograms (EGMs) acquired by a grid of point electrodes (the red asterisks in Video above) were computed. The EGMs were mapped back to the 3D geometry by using nearest neighbor interpolation. Two different configurations were used: 9x9 electrodes spaced by 3mm and 5x5 electrodes spaced by 6mm. The number of sampling sites affects the resolution of the reconstructed activation sequence.
Output of the 9x9 3mm catheter.
Output of the 5x5 6mm catheter.
A an algorithm was developed (Valinoti et al., Computing in Cardiology 2017) in order to detect the rotor rotational center from the acquired EGMs.
Computed rotor center trajectory.
This computational framework is being used to benchmark rotor-driven ablation. Find more about this project in the paper:
Martino Alessandrini, Maddalena Valinoti, Axel Loewe, Tobias Oesterlein, Olaf Dossel, Cristiana Corsi, Stefano Severi: A Computational Framework to Benchmark Basket Catheter Guided Ablation, proc. of Computing in Cardiology 2017, Rennes, FR.
A benchmarking framework for rotor-driven ablation of atrial fibrillation with multielectrode catheters
AF effectively alters intra atrial fluid dynamics in a manner that favors blood stasis and, hence, the chances of formation of thrombi. One factor is atrial enlargement: due to AF, the two atria progressively increase volume and sphericity. Another factor the elongation of the left atrial appendage, a dead-end structure attached to the left atrium which intrinsically favors blood stagnation. Another factor is chaotic contraction which reduces the exchange of blood. Surprisingly, none of these factors is taken into account by clinical stroke risk stratification indices. As a consequence, the predictive power of such indices remains low.The exact way these complex mechanisms interplay cannot be assessed experimentally and remains largely unknown. In this respect, computational fluid dynamics (CFD) represents a unique tool to test different boundary conditions on a complex fluid dynamics system, such as intra-cardiac hemodynamics, in a non-invasive fully controllable and reproducible way. The aim of this study was therefore to develop a patient-specific CFD model of the left atrium in atrial fibrillation which could help elucidate the role of the key anatomical and functional features of AF on the LA blood flow. By that, the provided tool might be used for improving personalized stroke risk stratification and therapy planning.
This study is the result of a collaboration between the Department of Electrical, Electronic and Information Engineering at UNIBO (Bologna, IT), MOX at Politecnico di Milano (Milano, IT) and Ospedale S. Maria delle Croci (Ravenna, IT).
A personalization pipeline was put in place to constrain the CFD problem with patient specific boundary conditions. Hereto, dynamic CT data was used to extract detailed atrial geometry and motion while PW Doppler was used to constrain the inflow at the four pulmonary veins.
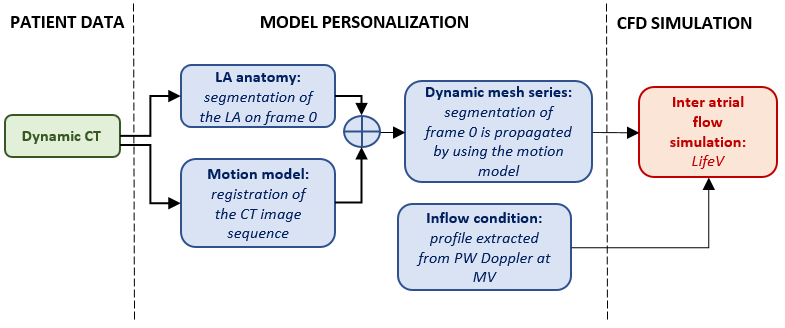
Model personalization for CFD simulations of atrial blood flow in AF.
This framework was used to develop personalized CFD models of two AF patients and to study the effect of a fibrillatory episode on the intra atrial fluid dynamics. CFD simulations were run with the life-v library. As such, a detailed reconstruction of intra atrial blood flow was calculated starting from personalized boundary conditions.
Output of the CFD simulation, produced with life-V.
The model showed that AF affects blood blow by reducing average blood velocity and vorticity. Moreover, blood was shown to remain longer in the atrial appendage than during sinus rhythm. This can explain the increased risk of thrombi.
More info at:
Alessandro Masci, Martino Alessandrini, Davide Forti, Filippo Menghini, Luca Dede, Corrado Tommasi, Alfio Quarteroni, Cristiana Corsi: A Patient-Specific Computational Fluid Dynamics Model of the Left Atrium in Atrial Fibrillation: Development and Initial Evaluation. In: Pop M., Wright G. (eds) Functional Imaging and Modelling of the Heart. FIMH 2017. Lecture Notes in Computer Science, vol 10263. Springer, Cham.
Related publications
- Alessandro Masci, Martino Alessandrini, Davide Forti, Filippo Menghini, Luca Dede, Corrado Tommasi, Alfio Quarteroni, Cristiana Corsi: A Patient-Specific Computational Fluid Dynamics Model of the Left Atrium in Atrial Fibrillation: Development and Initial Evaluation. In: Pop M., Wright G. (eds) Functional Imaging and Modelling of the Heart. FIMH 2017. Lecture Notes in Computer Science, vol 10263. Springer, Cham.
- Alessandro Masci, Martino Alessandrini, Davide Forti, Filippo Menghini, Luca Dede, Corrado Tommasi, Alfio Quarteroni, Cristiana Corsi, Design and Development of Computational Fluid Dynamics Model of the Left Atrium in Atrial Fibrillation on a Patient-Specific Basis, proc. of Computing in Cardiology 2017, Rennes, FR.
- Martino Alessandrini, Maddalena Valinoti, Axel Loewe, Tobias Oesterlein, Olaf Dossel, Cristiana Corsi, Stefano Severi: A Computational Framework to Benchmark Basket Catheter Guided Ablation, proc. of Computing in Cardiology 2017, Rennes, FR.
- M. Alessandrini, M. Valinoti, A. Pasini, R. Mantovan, S. Severi and C. Corsi: An automatic framework for the non-rigid alignment of electroanatomical maps and preoperative anatomical scans in atrial fibrillation, 2016 Computing in Cardiology Conference (CinC), Vancouver, BC, 2016, pp. 965-968.


